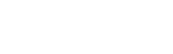ACIST HDi®
HD IVUS System
ACIST HDi®
HD IVUS System
ACIST HDi High-Definition IVUS System enables optimized visualization to inform and illuminate coronary and peripheral intervention strategy through advanced imaging modes, improved deliverability of the Kodama IVUS Catheter and interactive compact console.
Kodama®
Now indicated for both coronary and peripheral vascular procedures.

See more with ACIST HDi
Providing you the power to see with certainty.
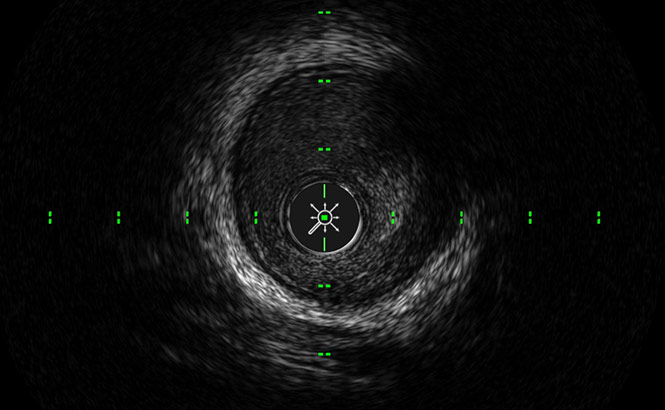
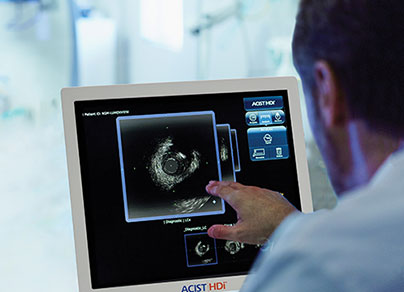
LumenView™
Darkens the coronary lumen for better border detection.
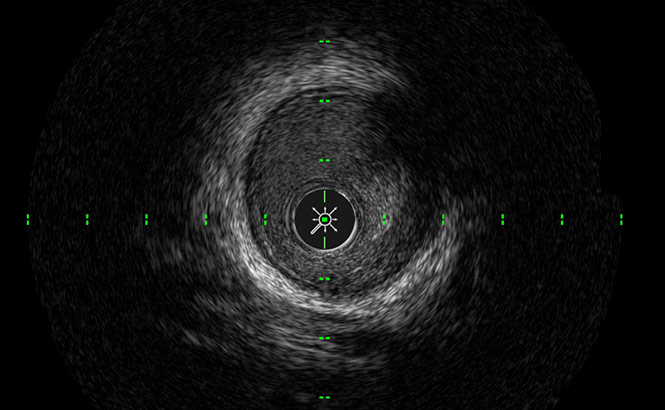
SilkView™
Increases gray scale for finer blood speckle, tissue and plaque differentiation.
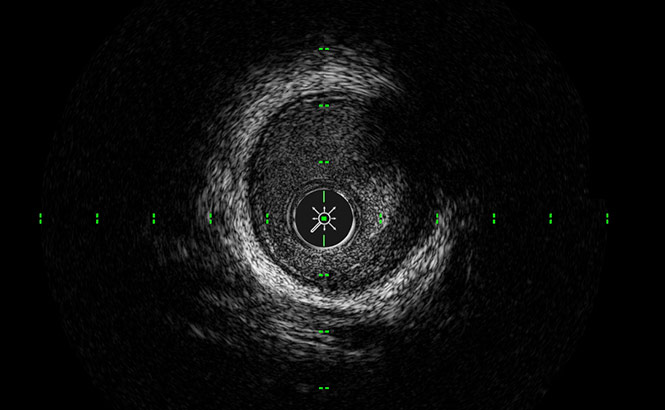
ClassicView™
Optimizes the balance of high resolution and depth of penetration and enables full vessel wall visualization.
Coronary IVUS
Better visualization of media than OCT for optimizing stent sizing4
Enables enhanced imaging by providing sufficient penetration at 60 MHz to see the media layer, even in larger plaque volumes4, so the physicians can maximize the stenting cross sectional area and may lead to better patient outcomes5

IVUS Use changed the treatment strategy during the procedure 74% of the time6
IVUS-guided stents also had low proximal & distal plaque burden, & higher MSA
compared to the angio-guided group7

8x more detection of lipid pools at 60 MHz than 40 MHz8
IVUS-guided PCI is beneficial for preventing the Stent Edge Restenosis in cases with diffused plaque lesions compared to angiography alone9

IVUS is less expensive and more cost effective than
angiography in 71% of PCI procedures10
IVUS provides improved outcomes at lower costs, with greater economic benefit especially in higher risk subgroups (diabetes, renal insufficiency, ACS)
This is based on a lifetime economic model from Italian healthcare payer perspective10
See the Difference. Visualize with Confidence.18
Peripheral IVUS

Use of IVUS in lower extremity procedures was associated with lower risk of complications post-procedure and lower risk of amputation11

2.8x less target lesion revascularizations at one-year follow up when using IVUS in directional atherectomy procedures compared to angiography alone12
Visual estimation measurements obtained from angiographic images were consistently smaller across all arterial segments than those obtained from IVUS assessment13
69% more calcium detected by IVUS compared to angiography in the same lesions14
2x more thrombus identified by IVUS compared to angiography15
4x more post-stent dissections were identified by IVUS compared to angiography16
3.5x more post-angioplasty dissections were identified by IVUS compared to angiography17
6x more post-atherectomy dissections were identified by IVUS compared to angiography17
See the Difference. Visualize with Confidence.18
Watch the Product Video
References
- Data on file – TR-07057 – Internal testing
- Data on file – TR-4050 – Study Summary for Kodama Catheter performance
- Data on file – TR-07057 – Internal testing
- IVUS-Guided Versus OCT-Guided Coronary Stent Implantation: A Critical Appraisal https://doi.org/10.1016/j.jcmg.2017.09.008
- Defining a new standard for IVUS optimized drug eluding stent implantation: the PRAVIO study. Catheter Cardiovasc Interv. August 1, 2009;74(2):348-356. 6. Maehara A, et al – 16 Nov 2018https://doi.org/10.1161/CIRCINTERVENTIONS.117.006243Circulation: Cardiovascular Interventions. 2018;11:e006243
- https://www.tctmd.com/news/ultimate-ivus-superior-angiography-guiding-pci-less-tvf-1-year. https://doi.org/10.1016/j.jacc.2018.09.01 8. Tanaka S, Sakamoto K, Kitahara H, et al. Assessments of lipid plaque and thrombus with a novel high-definition 60-MHz IVUS imaging system: comparison with conventional 40-MHz IVUS and OCT. J Am Coll Cardiol. 2013;62(18_S1):B201-B202 8a. Total of 50 matched cross-sections were analyzed with lipid core identified in 8 cross-sections in an ex-vivo study.
- Impact of the distance from the stent edge to the residual plaque on edge restenosis following DES implantation. PLOS One. 2015;10(3):E0121079. 10. Alberti, A., Giudice, P., Gelera, A. et al. Understanding the economic impact of intravascular ultrasound (IVUS). Eur J Health Econ 17, 185–193 (2016). https://doi.org/10.1007/s10198-015-0670-4
- Panaich, S. S., Arora, S., Patel, N., Patel, N. J., Savani, C., Patel, A., … Badheka, A. O. (2016). Intravascular Ultrasound in Lower Extremity Peripheral Vascular Interventions: Variation in Utilization and Impact on In-Hospital Outcomes From the Nationwide Inpatient Sample (2006–2011). Journal of Endovascular Therapy, 23(1), 65–75. https://doi.org/10.1177/1526602815620780
- Krishnan, P., Tarricone, A., K-Raman, P., Majeed, F., Kapur, V., Gujja, K., … Sharma, S. (2018). Intravascular ultrasound guided directional atherectomy versus directional atherectomy guided by angiography for the treatment of femoropopliteal in-stent restenosis. Therapeutic advances in cardiovascular disease, 12(1), 17–22. doi:10.1177/1753944717745509
- Pliagas, G., Saab, F., Stavroulakis, K., Bisdas, T., Finton, S., Heaney, C., … Mustapha, J. A. (2020). Intravascular Ultrasound Imaging Versus Digital Subtraction Angiography in Patients With Peripheral Vascular Disease. The Journal of Invasive Cardiology, 32(3), 99–103. Retrieved from https://www.invasivecardiology.com/articles/intravascular- ultrasound-imaging-versus-digital-subtraction-angiography-patients-peripheral-vascular-disease?fbclid=IwAR1qh_GQ85jMvJqGpOeYU_So2gaYF7ol5sknbJmoO-GMmB8JjvMd7gscSi0
- Yin D et al. Intravascular Ultrasound validation of contemporary angiographic scores evaluting the severity of calcification in peripheral arteries. J Endovasc Ther 2017; 24:478-87. 15. Shammas et al. Dethrombosis of the lower extremety arteries using the power-pulse spray technique in patients…. J Endovasc Ther 2008;15:570-79. 16. Miki K, Fujii K, Fukunaga M, et al. Impact of post-procedural intravascular ultrasound findings on long-term results following self-expanding nitinol stenting in superficial femoral artery lesions. Circ J 2013; 77:1543-1550. 17. Shammas NW, Torey JT, Shammas WJ, Jones-Miller S, Shammas GA. Intravascular ultrasound assessment and correlation with angiographic findings demonstrating femoropopliteal arterial dissections post atherectomy: results from the iDissection study. J Invasive Cardiol. 2018;30:240–244.
- Krishnan, P., Tarricone, A., K-Raman, P., Majeed, F., Kapur, V., Gujja, K., … Sharma, S. (2018). Intravascular ultrasound guided directional atherectomy versus directional atherectomy guided by angiography for the treatment of femoropopliteal in-stent restenosis. Therapeutic advances in cardiovascular disease, 12(1), 17–22. doi:10.1177/1753944717745509
- Pliagas, G., Saab, F., Stavroulakis, K., Bisdas, T., Finton, S., Heaney, C., … Mustapha, J. A. (2020). Intravascular Ultrasound Imaging Versus Digital Subtraction Angiography in Patients With Peripheral Vascular Disease. The Journal of Invasive Cardiology, 32(3), 99–103. Retrieved from https://www.invasivecardiology.com/articles/intravascular-t sound-imaging-versus-digital-subtraction-angiogra phy-patients-peripheral-vascular-disease?fbclid=IwAR1qh_GQ85jMvJqGpOeYU_So2gaYF7ol5sknbJmoO-GMmB8JjvMd7gscSi0
- Yin D et al. Intravascular Ultrasound validation of contemporary angiographic scores evaluting the severity of calcification in peripheral arteries. J Endovasc Ther 2017; 24:478-87.
- Shammas et al. Dethrombosis of the lower extremety arteries using the power-pulse spray technique in patients…. J Endovasc Ther 2008;15:570-79.
- Miki K, Fujii K, Fukunaga M, et al. Impact of post-procedural intravascular ultrasound findings on long-term results following self-expanding nitinol stenting in superficial femoral artery lesions. Circ J 2013; 77:1543-1550.
- Shammas NW, Torey JT, Shammas WJ, Jones-Miller S, Shammas GA. Intravascular ultrasound assessment and correlation with angiographic findings demonstrating femoropopliteal arterial dissections post atherectomy: results from the iDissection study. J Invasive Cardiol. 2018;30:240–244.’ 8. Data on file – TR-07057 – Internal testing. 9. Data on file – TR-4050 – Study Summary for Kodama Catheter performance.
- Data on file – TRCO-17355
Rx Only
Prior to use, reference Instructions for Use, inside the product carton (when available) or at < enterwebsite.com> for more detailed information on safe use of the device.
Indications for Use:
The ACIST HDi® System is intended to be used for the ultrasound examination of coronary and peripheral intravascular pathology. Intravascular ultrasound imaging is indicated in patients who are candidates for transluminal interventional procedures. The ACIST Kodama Intravascular Ultrasound Catheter is intended for use with the ACIST HDi System.
Contraindications:
Contraindicated for patients with: bacteremia or sepsis; arterial spasm; major coagulation system abnormalities; mechanical heart valves that would be crossed by the catheter; severe hemodynamic instability or shock; total vessel occlusion (prior to initial stages of revascularization). Contraindicated for use in the cerebrovascular arteries. In coronary procedures, the product is also contraindicated for patients who are: disqualified for revascularization surgery; disqualified for balloon angioplasty (PTCA).
Important Safety Info:
Intravascular ultrasound studies using this product should be performed only by physicians and other medical professionals fully trained in the required techniques and procedures.The Kodama catheter contains a short monorail guidewire engagement system. As such, it is susceptible to guidewire entanglement and/or prolapse during catheter deployment and withdrawal. Before use and when possible during use, inspect the Kodama catheter carefully for kinks or any other damage. Do not use a kinked or damaged catheter because vessel damage and/or the inability to advance or withdraw the catheter may occur.
Never advance or withdraw the Kodama catheter against resistance until the cause of the resistance is determined by fluoroscopy. Movement of the catheter or guidewire against resistance may result in elongation or separation of the catheter or guidewire tip, damage to the catheter, or vessel perforation.
When advancing the Kodama catheter through a stented vessel, short monorail catheter designs are susceptible to guidewire/ catheter entrapment, catheter tip separation, and/or stent dislocation.
Adverse events that may occur as a consequence of intravascular ultrasound imaging include (but are not limited to): vessel occlusion and/or abrupt closure; air embolism; vessel dissection, injury, or perforation; vessel rupture, injury, or perforation; acute myocardial infarction; cardiac arrhythmias including but not limited to ventricular tachycardia, ventricular fibrillation, and complete heart block; cardiac tamponade; catheter/guidewire entrapment; catheter induced ischemia; death; vessel trauma requiring treatment/surgical intervention, including angioplasty/stent; infection; stent strut damage; stroke (including cerebral vascular accident and transient ischemic attack); thrombus formation or thromboembolism; vasospasm.