ACIST Diastolic Pressure Ratio (dPR)
Non-hyperemic index for coronary physiology
ACIST Diastolic Pressure Ratio (dPR)
Non-hyperemic index for coronary physiology
ACIST diastolic pressure ratio (dPR), using the ACIST RXi® Rapid Exchange System and Navvus® II MicroCatheter, provides a non-hyperemic alternative for physiological assessment of coronary disease.
Reducing costs, time and patient discomfort
Reduced Patient Discomfort
Reduced Cost
Reduced Time
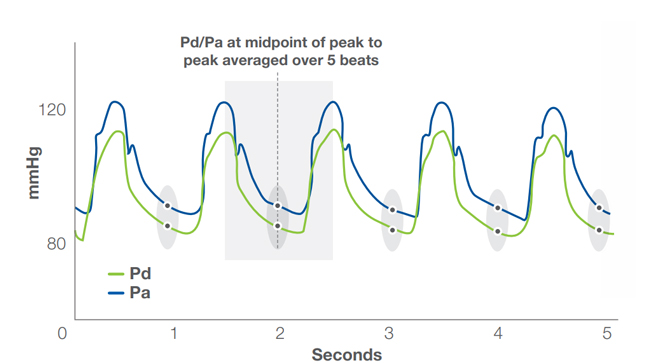
ACIST dPR algorithm
ACIST dPR by the numbers
(compared to iFR)2
0.89
dPR cutpoint
99.68%
Sensitivity
88.92%
Specificity
0.89
dPR cutpoint
0.993
AUC
99.68%
Sensitivity
88.92%
Specificity
0.89
dPR cutpoint
0.993
AUC
98.68%
Sensitivity
88.92%
Specificity
98.3%
PPV
99.2%
NPV
Analysis of the ACIST FFR Study1
Analysis of ACIST dPR vs. iFR3
Purpose

Methods
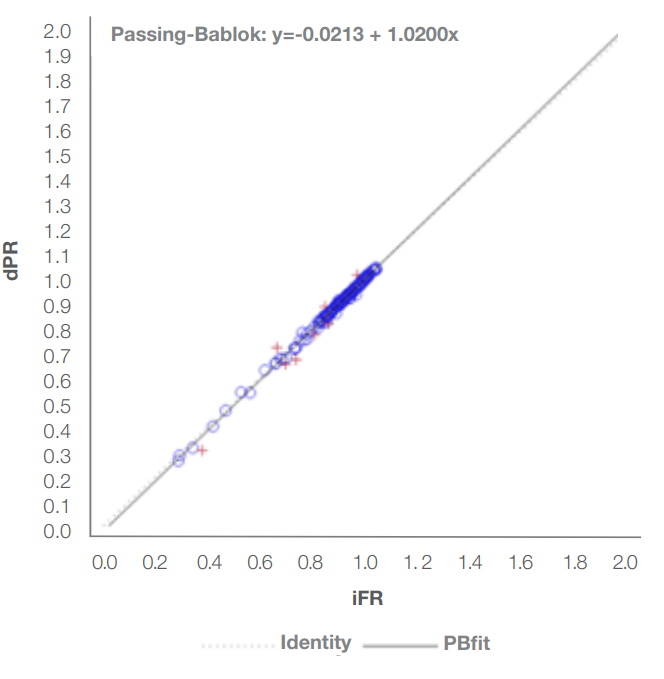
Key points
- ACIST dPR is highly correlated with iFR
- ACIST dPR provides similar diagnostic accuracy as iFR
Results
Diagnostic accuracy of dPR (cutpoint of 0.89) referenced to iFR (0.89) was 93.89%
Rx Only
* Reduced side effect profile when comparing resting approach (iFR, dPR, Pd/Pa) to FFR with adenosine induced hyperemia
** Cost savings based on the reduced cost of utilizing a resting approach compared to conventional FFR and respective cost of admInistration of hyperemic agent (adenosine)
*** When comparing resting index (iFR, dPR, Pd/Pa) to FFR with adenosine induced hyperemia
1. Data on file TR-07879
2. Sen S, Escaned J, Malik IS, et at. Development and validation of a new adenosine-independent index of stenosis severity from coronary wave-intensity analysis: results of the ADVISE (ADenosine Vasodilator Independent Stenosis Evaluation) study. J Am Coll Cardbl. 2012:59(15)1392-1402. doi:10.1016/j.jacc.2011.11.003.
ACIST, ACIST FiXi and Nawus are trademarks of ACIST Medical Systems, Inc. ACIST Medical Systems, Inc., reserves the right to modify the specifications and features described herein, or discontinue manufacture of the product described at any time without prior notice or obligation. Please contact your authorized ACIST representative for the most current information.
© 2020 ACIST Medical Systems, Inc. Al Rights Reserved.
* Reduced side effect profile when comparing resting approach (iFR, dPR, Pd/Pa) to FFR with adenosine induced hyperemia
** Cost savings based on the reduced cost of utilizing a resting approach compared to conventional FFR and respective cost of administration of hyperemic agent (adenosine)
*** When comparing resting index (iFR, dPR, Pd/Pa) to FFR with adenosine induced hyperemia
1. Data on file TR-14743
2. Johnson NP, Jeremias A, Zimmermann FM, et al. Continuum of Vasodilator Stress From Rest to Contrast Medium to Adenosine Hyperemia for Fractional Flow Reserve Assessment. JACC Cardiovasc Interv. 2016;9(8):757-767. doi:10.1016/j.jcin.2015.12.273.
ACIST, ACIST RXi and Navvus are trademarks of ACIST Medical Systems, Inc. ACIST Medical Systems, Inc., reserves the right to modify the specifications and features described herein, or discontinue manufacture of the product described at any time without prior notice or obligation. Please contact your authorized ACIST representative for the most current information.
© 2024 ACIST Medical Systems, Inc. All Rights Reserved. RXi-XXXXXXX_US 07/24
1. Kubota M, Oguri A. Diagnostic accuracy of diastolic pressure ratio using a pressure microcatheter for intracoronary physiological assessment. Heart Vessels. 2023 Dec;38(12):1395-1403. doi: 10.1007/s00380-023-02301-5. Epub 2023 Aug 26. PMID: 37626238.
2. 510k K233904. Cleared July 17, 2024. As is the case with any physiologic measurement and the use of non-hyperemic indices (Pd/Pa, dPR), the physician should also consider patient history and use medical expertise and clinical judgment to determine if an additional measurement of FFR during hyperemia and/or therapeutic intervention are indicated.
3. Data on file TR-14743.
4. Johnson NP, Jeremias A, Zimmermann FM, et al. Continuum of Vasodilator Stress From Rest to Contrast Medium to Adenosine Hyperemia for Fractional Flow Reserve Assessment. JACC Cardiovasc Interv. 2016;9(8):757-767. doi:10.1016/j.jcin.2015.12.273.
The ACIST RXi® System is indicated for obtaining intravascular pressure measurements for use in the diagnosis and treatment of coronary and peripheral artery disease. The ACIST Navvus® MicroCatheter is intended for use with the ACIST RXi System.
ACIST, ACIST RXi and Navvus are trademarks of ACIST Medical Systems, Inc. ACIST Medical Systems, Inc., reserves the right to modify the specifications and features described herein, or discontinue manufacture of the product described at any time without prior notice or obligation.
Please contact your authorized ACIST representative for the most current information.
© 2024 ACIST Medical Systems, Inc. All Rights Reserved. BMT-RXi-2400017_US 09/24